The impact of Brexit: from the CE to the UKCA mark
Published by Valeria Minasi. .
Brexit Export Export markets Market AccessibilityUKCA mark is the new mark to be used for the goods placed on the British market. It is going to cover many goods on which the CE marking was affixed beforehand. Anyway, CE marking remains still valid for Northern Ireland1.
Today, technical requirements, procedures and assessment standards used to demonstrate compliance and to obtain the UKCA mark seems to be identical in substance to that required for the authorisation of use of the CE mark. The existing stock, i.e. the goods stocked within the 31st December 2020, ready to be placed on the market and already bearing the marking and the details of the certifying body, is approved to be distributed in Great Britain with the CE mark until the end of 2021, although this mark was released by a UK body EU approved.
Compliance certifying bodies
The approved bodies settled in Great Britain will henceforth be UK approved bodies. In fact, the UK approved bodies will lose the legal status of EU approved bodies and will be deleted out of the European Commission information system for the certifying bodies. Since the 1stJanuary, these bodies may not carry out any assessment procedure on the basis of the EU rules on products. The EC Certificates of Conformity issued by these bodies should have been forwarded to an EU 27 Approved Body within the 31st December 2020 to maintain their validity. The UK BEIS Department instituted a list of the Approved Bodies in Great Britain. The EU-type Examination certificate issued by the EU approved body will be approved in GB until the 31st December 2021. Not being anticipated recognition agreements between EU and GB which would permit bodies to operate in both the schemes, a UK body may certify only the UKCA mark, while an EU certifying body may certify the CE mark. The UK approved bodies with competence for MDD, IVDD or AIMDD may have the automatic renewal of their notifications, without having to comply with a new designation process. Furthermore, from January, the authorised representatives and the persons responsible for the certification based in the EU are not even recognised in Great Britain. Nowadays, the UK bodies authorised to issue the UKCA certification are the UKCAS accredited bodies2.
Transitional measures for the UKCA marking
- 2021: given that the provisions prescribed by the EU and the UK laws remain the same, it is possible to place a CE marked product in Great Britain if one of the following conditions recurs:
- the CE mark is based on a self-declaration;
- the conformity assessment has been issued by an EU approved body;
- the technical documents needed for the conformity certificate, issued by a UK approved body, have been forwarded to an EU approved body. Anyway, the companies may start to use the UKCA marking immediately.
- 2022: UKCA mark will become mandatory. Since 1st January 2022, the UK authorities will not recognise the CE mark for entering the British market. Goods exhibiting both the CE and the UKCA mark will be accepted, provided that they comply with both the British and the European rules.
- 2023: indelibly UKCA marking on the product.
How to use the UKCA marking
- MARK POSITIONING. It is often necessary to apply the UKCA marking to the product itself or to its package. The following general rules are applied:
- UKCA marks can be exposed on the goods only by the manufacturer or the authorised representative;
- when the UKCA marking is applied, the manufacturer (or the authorised representative) is responsible for the compliance of the goods to the requirements of the relative law;
- it is necessary to use the UKCA mark only to demonstrate the compliance of the product to the rules of the United Kingdom;
- no mark should be added to avoid a wrong interpretation of the meaning or of the shape of UKCA mark to others;
- do not affix any labels on the product: they could affect the visibility, the clarity or the interpretation of the UKCA mark;
- UKCA marking should not been affixed on the goods if not expressly required.
- RULES FOR THE USE OF THE IMAGE. You must take sure that:
- if the marking dimensions are reduced or augmented, the set of characters have to be proportioned;
- the UKCA marking must be 5mm height unless another minimum size is specified in the relevant legislation;
- the UKCA marking must be clearly legible.
- TECHNICAL DOCUMENTS. The manufacturer or the authorised representative in the United Kingdom must keep the documents in order to demonstrate that the product complies with the law requirements. The records to be retained may vary depending on the specific regulations related to the category of goods. It is necessary to keep the general registrations about:
- product's projecting and manufacturing;
- compliance of the product to the relevant requirements;
- address of the manufacturer and, eventually, of any storage buildings.
Declaration of conformity of the United Kingdom
The Declaration of Conformity of the United Kingdom is a document requested for most of the products with a UKCA mark. The declaration of conformity of UK must be available to the supervisory bodies if requested. The information required on the declaration of conformity is to a very large extent identical to that required for the EU declaration of conformity. Furthermore, it is necessary to state the compliance to: a) the specific Uk rules, rather than the EU; b) UK standards rather than standards related in the EU Official Journal.
Product areas concerned by the UKCA mark
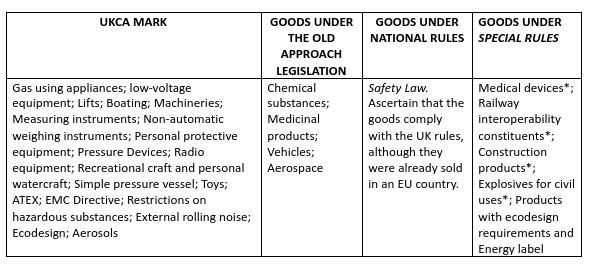
* Medical devices. Since the 1st January 2021, roles and responsibilities of the manufacturer and the supplier of medical devices and of IVD have been changed. Manufacturers who want to sell a device on the UK market must first register to the MHRA. If the manufacturing company is not based in the UK, a Responsible Person in the UK to undertake the registration and to act on his behalf. Since the 1st January 2021, under the MDR 2002 (Medical Devices Regulations 2002), a product CE marked with a declaration of conformity or a valid certification will be considered as compliant to the requirements of UKCA marking and the CE mark will continue to be recognised in Great Britain until 30th June 2023. Since the 1stJanuary 2021, medical devices placed on the UK market should have a UKCA mark or a CE marking, depending on the certification applied to the product.
* Railway Interoperability. The operator established in the EU must require the documents to the ORR (Office of Rail and Road) to execute his services in Great Britain. The UK will recognise the assessment documents of the EC Conformity with respect to the technical railway characteristics of the United Kingdom. Since the 1st January 2021, the placement of interoperability constituents in the UK drew on an assessment process of the conformity in the United Kingdom, requiring the compliance with the National Technical Specifications Notices (NTSN). If the requirements in the NTSN are identical to the Technical specifications of Interoperability (STI) of EU, an interoperability constituent can be sold with the assessment documents of EC conformity with respect to the relative requirements. Vehicles authorised for the first time in the UK, since the 1st January 2021, must obtain an EU authorisation before their usage in the Union too.
* Construction Products. The legislation has to be adopted by the Parliament, therefore the following statements are subject to changes. The operators must prepare for the termination of the recognition of CE marking in the UK and affix the UK marking appealing to an “approved body” recognised in the UK within the 1st January 2022. Some of the CE marked goods, satisfying the EU requirements, can be still placed in the UK market until the 1st January 2022. All European harmonised requirements are going to become “designated standards” in the UK. Following the withdrawal agreement provisions, goods legally CE marked and placed on the EU market before the transition period, can continue to run until it reaches its end user, even in the UK or in the EU.
* Explosives for Civil Uses. Explosives for civil uses with a CE mark can be placed on the Uk market until the 1st January 2022. Since the 1st January 2022 UK marking has to be used. Until the 31st December 2022 the UKCA mark must be affixed directly on the goods or on an accompanying document. The Explosives Notified Body (ENB) still is the Approved Body in the UK to assess the conformity of the explosives for civil uses.


